Several properties of coatings are the subject of improvement. Often, those properties are directly related to the composition of the resin system and the specific application of the paint. It turns out that polysiloxanes, often called silicones, can be used to improve a variety of coating properties. This article is about improving the outdoor durability of coatings by combining organic resins and polysiloxane1.
Outdoor durability
Outdoor durability of coatings, often called weatherability or exterior durability, refers to the resistance against degradation during outdoor exposure. Photo-oxidation, caused by the UV component of electromagnetic radiation from the sun, and hydrolysis of the resin system are important processes during the deterioration of coatings during outdoor exposure2. A coating can yellow, crack, lose gloss, or lose adhesion because of degradation.

Cracking and loss of adhesion of a coating caused by exterior exposure.
The speed of degradation of a coating during outdoor exposure depends, amongst others, upon two key factors. First, the strength of the bonds in the resin system is important. The higher the bond energy, the more difficult it is to break the system down. Secondly, the degree of UV absorption by the resin governs how many radicals can be formed during exposure of the coating to sunlight. Radicals act as the ‘scissors’ for chemical bonds during photo-oxidative degradation of coatings.
Most resins that are used for paints, like alkyds, polyesters, polyacrylics and epoxies, are organic polymers that are based on carbon (C) chemistry. Even though biobased raw materials become more important, most of the raw materials used to make organic resins come from fossil sources. Organic resins have limited resistance against UV radiation, heat and hydrolysis.
Key properties of polysiloxane
When looking at durability, an interesting alternative for pure organic resins is to use inorganic polymers that are based on silicon (Si) chemistry, like polysiloxanes.
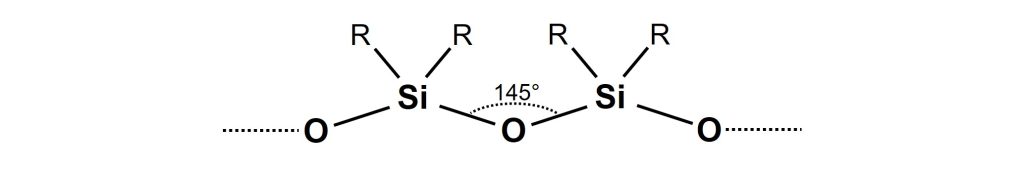
General structure of linear polysiloxane.
The backbone of polysiloxanes consists of silicon and oxygen atoms. The side groups (R) are organic groups, most often methyl and/or phenyl.

Key characteristics of polysiloxanes, compared to organic polymers.
Two key properties of polysiloxanes can be directly linked to the excellent outdoor durability of these polymers. First, the bonds in the polymer backbone are strong, implying that a high amount of energy is needed to break the backbone down. Secondly, polysiloxane is nearly transparent to UV radiation.
How to use polysiloxane in coatings?
The concept that is often used, when durability must be improved, is that part of the organic resin is substituted by polysiloxane. The durability of a resin improves when the polysiloxane content is increased. Polysiloxanes of low molecular weight, so-called polysiloxane oligomers, can be used as building blocks in the synthesis of resins that are used in paints. The branched, 3-dimensional polysiloxanes contain functional groups, most often hydroxyl (–OH) and/or methoxy (–OCH3) groups, that can react with hydroxyl groups of organic resins. Typically, the co-condensation of the organic and inorganic polymers is done in a reactor at around 150 °C.
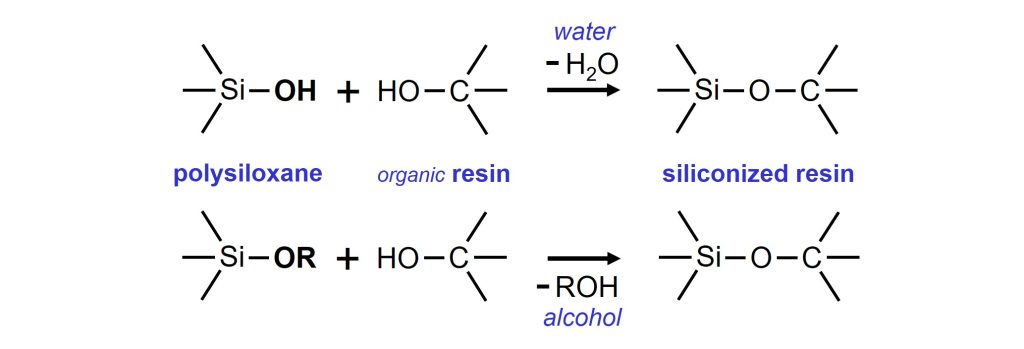
Co-condensation reactions of polysiloxane with organic resins.
Alkyd resins, polyester resins, epoxies and polyacrylics with improved durability can be made in this way. The resulting resins are said to be inorganic-organic hybrid resins. The more polysiloxane is used, the higher the inorganic content of the resin is and the better the resistance against UV radiation, heat and hydrolysis becomes. The polysiloxane content of siliconized alkyd resins, used for exterior architectural paints, is typically in the range of 15 to 35 weight-%.
An example:
Several companies supply polysiloxane oligomers that can be used as building block in the synthesis of resins to obtain hybrid binders with improved durability. SILRES® IC 368 of Wacker Chemie AG, being a global leader in silicon chemistry, is an example of such a product.

Key characteristics of SILRES® IC 368.
SILRES® IC 368 is a solvent-free liquid polysiloxane oligomer, that can be used as building block to modify organic polymers, such as alkyds, polyesters and polyacrylics. During synthesis, the methoxy groups (-OCH3) of the polysiloxane react with hydroxyl groups (-OH) of the organic resin. The resulting siliconized resins, being inorganic-organic hybrid polymers, can be used as binders for coatings that have improved resistance against weathering, hydrolysis and heat.
References
- Silicone Resins and their Combinations, Wernfried Heilen & Sascha Herrwerth, Vincentz Network, 2015.
- Cause and Defect: Evaluating and Testing the Weathering of Coatings, Ron Lewarchik, 15 February 2019.
The views, opinions and technical analyses presented here are those of the author or advertiser, and are not necessarily those of ULProspector.com or UL Solutions. The appearance of this content in the UL Prospector Knowledge Center does not constitute an endorsement by UL Solutions or its affiliates.
All content is subject to copyright and may not be reproduced without prior authorization from UL Solutions or the content author.
The content has been made available for informational and educational purposes only. While the editors of this site may verify the accuracy of its content from time to time, we assume no responsibility for errors made by the author, editorial staff or any other contributor.
UL Solutions does not make any representations or warranties with respect to the accuracy, applicability, fitness or completeness of the content. UL Solutions does not warrant the performance, effectiveness or applicability of sites listed or linked to in any content.
