This topic has been covered many times over the last eight years and previous articles should be reviewed to better understand the topic. At the end of this article, you will find a list of related articles that can be found in the Prospector® Knowledge Center for further reading. The fact that there are so many articles just shows the emphasis on the topic and how important dispersion is to many materials in industry. Dispersants are used in paints, inks, adhesives and cosmetics, etc. Anywhere you are separating or diffusing solid particles in a liquid medium or paste.
In previous articles, topics like “Surfactants,” and “The Differences Between Wetting Agents and Dispersants ” have been discussed. Here, I solely present the subject of dispersants. If there is any confusion between the roles of wetting agents and dispersants, I suggest you read the latter article.
The definition of dispersant, in terms of physical chemistry, is a material that, when incorporated into an admixture, is capable of homogeneously maintaining the dispersed particles in suspension. Therefore, the goal is to distribute dry pigments into a liquid medium and eliminate agglomerates.
Agglomerates are clumps of pigment, which, when not dispersed fully, affect many properties including[1]:

Figure 1. Pigment rub-out with flocculation
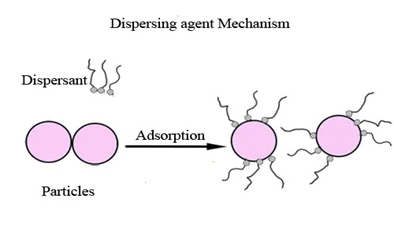
Figure 2. Dispersion of Flocculates
- Flocculation
- Flooding/floating
- Bénard cells
- Color shift/change
- Gloss decrease
- Hiding
The most important of these depending on your paint system, is hiding, gloss and color acceptance. If the pigments are agglomerated it will decrease hiding, most likely reduce gloss and in most cases affect color acceptance.
ULTRUS helps companies work smarter and win more, with powerful software to manage regulatory, supply chain and sustainability challenges. Learn more here!
Several types of additives can be used in the dispersion process in which solid particles, like pigments and fillers, are distributed and stabilized in a liquid. It is not enough to separate the particles, since they can re-agglomerate afterwards. This re-agglomeration is termed flocculation. Flocculation can cause immediate problems with color, or perhaps it won’t be seen until application such as rolling out a paint in one area and brushing it out in another where there are different shear rates. Rolling/brushing color differences it’s called hat banding.
In Figure 1, a light blue paint has been applied to a substrate. Due to poor dispersion or flocculation, when the paint is partially dry, a test for dispersion is the application of shear by a finger in a circular motion (“rub-out test”). That energy in a semi-set paint film is sufficient to redistribute the pigment in the medium, resulting in the dark blue area. In addition to the dispersion of pigments, the surfactant displaces the air at the interface of the pigment particle. One of the more difficult materials to disperse has been single-walled carbon nanotubes, due to their morphology, and the strong attraction between the spindle-like particles which possess a high aspect ratio.
Dispersants absorb onto the pigment surface and therefore maintain pigment spacing by electrostatic repulsion and/or steric hindrance. Both stabilization mechanisms are described below.
Electrostatic Repulsion
The pigment particles in the liquid paint carry electrical charges on their surfaces. Ideally, through the use of additives, it is possible to make all pigment particles equally charged. Counterions to the pigment-surface ions, concentrate at the pigment surfaces (in the liquid phase) so that an “electrical double layer” is formed (Figure 3). This is the ideal case and never wholly occurs practically.
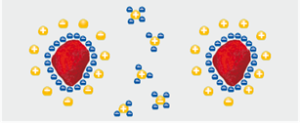
Figure 3. Electrical double layer on pigment surfaces
Steric Stabilization/Hindrance
Dispersing additives, which function by steric hindrance, display two structural features. First, the products contain resin-compatible chains (hydro-carbon units) which, after adsorption of the additive onto the pigment surface, project into the surrounding resin solution. This layer of adsorbed additive molecules with the protruding chains is referred to as steric hindrance or “entropic stabilization” (Figure 4).
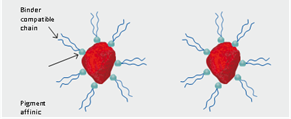
Figure 4. Pigment Stabilization through Steric Stabilization
Second, these materials contain one or more groups that have a strong affinity to attach to the pigment, also known as “anchor groups” – that all together provide a strong, durable adsorption onto the pigment surface.

Steric stabilization occurs in solvent-borne systems and in water-reducible systems which contain solvated resins. Structural features composed of pigment affinic groups (polar) and resin-compatible chains (non-polar), these additives exhibit surface-active properties. Therefore, they not only stabilize the pigment dispersion, but they also function as wetting agents.
The first question to answer when selecting your dispersing agent is whether you need electrostatic or steric repulsion. The answer is highly dependent on the system you formulate: Is it solvent-based? Water-based?
If your paint medium is a polar (solvent-based paint), the answer is simple. Electrostatic stabilization is impossible. Steric stabilization is the only way.
With a polar medium (water-based and some solvent-based formula),
- Electrostatic stabilization is the default way. This is for example the kind of stabilization mechanism that you would find in wall paints.
- In the case of more complex systems, steric stabilization can prove useful.
Again, dispersing agents are one of the more important additives to a paint system. Take the time to evaluate them and run some simple tests to decide what is the best option for you.
As mentioned earlier related articles are below for further information on dispersants. In addition, most equipment manufacturers of dispersing equipment also have short videos to show how their equipment works, and at the same time show the mechanism of dispersion.
Understanding Dispersants, 2016, Marc Hirsch;
Mixing Things Up: Formulating with Dispersants, 2018, George Deckner – Cosmetic Dispersants: function, mechanisms, types and formulation tips.
Dispersants – The Understood Misunderstood Additive, 2021 Marc Hirsch
Dispersants for Steric Stabilization, Dispersants for Electrostatic Stabilisation – Using dispersants for electrostatic stabilization in coating formulations, and The Differences between Wetting Agents and Dispersants, 2020 – 2022, Jochum Beetsma
There is also a good article in the Paint & Coatings Industry, Introduction to Additives, Part 4: Dispersants, 2021-12-14 PCI Magazine
The views, opinions and technical analyses presented here are those of the author or advertiser, and are not necessarily those of ULProspector.com or UL Solutions. The appearance of this content in the UL Prospector Knowledge Center does not constitute an endorsement by UL Solutions or its affiliates.
All content is subject to copyright and may not be reproduced without prior authorization from UL Solutions or the content author.
The content has been made available for informational and educational purposes only. While the editors of this site may verify the accuracy of its content from time to time, we assume no responsibility for errors made by the author, editorial staff or any other contributor.
UL Solutions does not make any representations or warranties with respect to the accuracy, applicability, fitness or completeness of the content. UL Solutions does not warrant the performance, effectiveness or applicability of sites listed or linked to in any content.

 ) as the applications and development manager in Core R&D in the Coatings & Functional Polymers Group. He also managed the TS&D group for coatings while at Dow Chemical (1995-99) and held positions at Rhodia (Laboratory Manager, Latex & Specialty Polymers (1989-95)) and was the Development Chemist, exterior latex paints at Benjamin Moore & Co. (1979-89).
) as the applications and development manager in Core R&D in the Coatings & Functional Polymers Group. He also managed the TS&D group for coatings while at Dow Chemical (1995-99) and held positions at Rhodia (Laboratory Manager, Latex & Specialty Polymers (1989-95)) and was the Development Chemist, exterior latex paints at Benjamin Moore & Co. (1979-89).