 Introduction
Introduction
Dispersing solid particles, like pigments and fillers, in a liquid is an important process in the production of paints and inks. Several types of additives can be used in the dispersion process, in which solid particles are wetted, separated from each other and stabilized in a liquid.
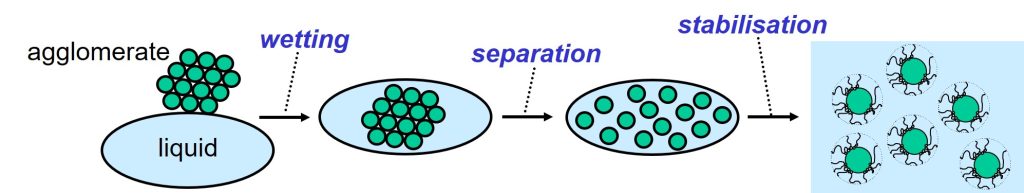
The three steps in the dispersion process.
Two categories of such additives, wetting agents and dispersants, are often mentioned in one breath. In documents, such as technical data sheets, an additive is often characterized as Wetting & Dispersing Additive. However, the two types of additives differ strongly with respect to the role they play in the process and with respect to the chemical composition and structure of the molecules the additives are composed of.
Function
A system developer, like a paint formulator, must have a clear view on what each component of a paint or ink should do. The job a raw material, like an additive, must do in a system is called its function. The function describes in a few words what the system developer would like the additive to do. A check question to ask if you are not sure about the function of a component in your system: What will happen if I remove the additive from the formulation?
Explore the ULTRUS Portfolio supporting regulatory, supply chain and sustainability needs. Learn more here!
Wetting agents
Wetting is the first step in the dispersion process. The solid raw materials that are used in paints and inks, pigments and fillers, are often supplied as powders. The solid particles are glued together in clusters, called agglomerates. During wetting, the air that surrounds the particles in an agglomerate, is substituted by liquid.
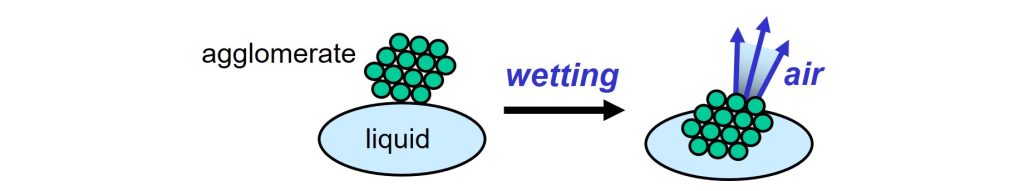
Wetting of solid particles: air is replaced by liquid.
Wetting will take place when the surface tension of the liquid is low compared to the surface energy of the solid particles1. Wetting will not occur when the surface tension is too high. The surface tension can be lowered by adding a wetting agent. This additive does its job because the molecules adsorb and orient at the liquid-air interface.
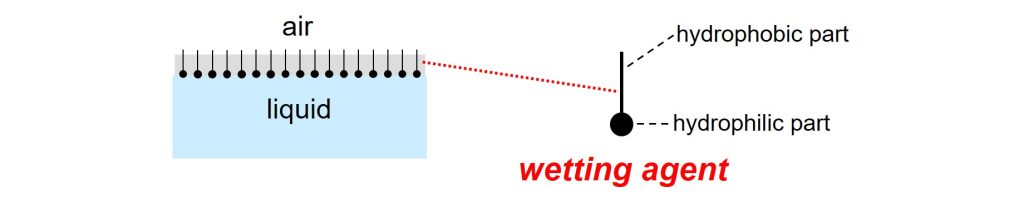
Wetting agent molecules adsorb and orient at a liquid-air interface.
Wetting agents show this behavior because the molecules consist of two parts. The hydrophobic part points towards the air and the hydrophilic part sticks into the liquid. An interface between a liquid and air is often referred to as a ‘surface’. Wetting agents are therefore often called surfactants, ‘agents’ that are active at a surface2.
There is a wide variety of wetting agents that are used in paints and inks.
Dispersants
Solid particles attract each other. Energy is therefore needed to separate the particles from each other in the second step of the dispersion process. Particles in a liquid will move to each other and glue together because of the attractive forces. The spontaneous and undesired gluing together of solid particles in a liquid is called flocculation. Solid particles that have been separated from each other are stabilized against flocculation by using a dispersant3,4. These additives do their job because the molecules are designed to adsorb at the solid-liquid interface and assure repulsion between the particles. The repulsive forces, provided by the dispersant, must be stronger than the attractive forces.
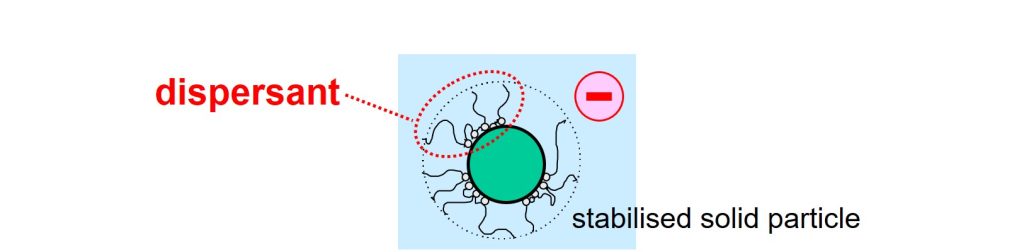
Dispersant molecules adsorbed at the interface of a solid particle and liquid.
Repulsion can result from two mechanisms that may be used separately or in combination. Particles repel each other when they all carry a charge of the same sign. This so-called electrostatic stabilization is obtained when the dispersant molecules carry a charge. Steric stabilization is obtained when all particles are covered with tails of sufficient length that dissolve in the liquid that surrounds the particles. The soluble tails are part of the dispersant molecules.
Many companies develop and supply dispersants for water-based, solvent-based and solvent-free systems.
Overview
Wetting agents and dispersants are additives that are used in dispersion processes. Both are interface additives, but there are fundamental differences between the two categories of materials.
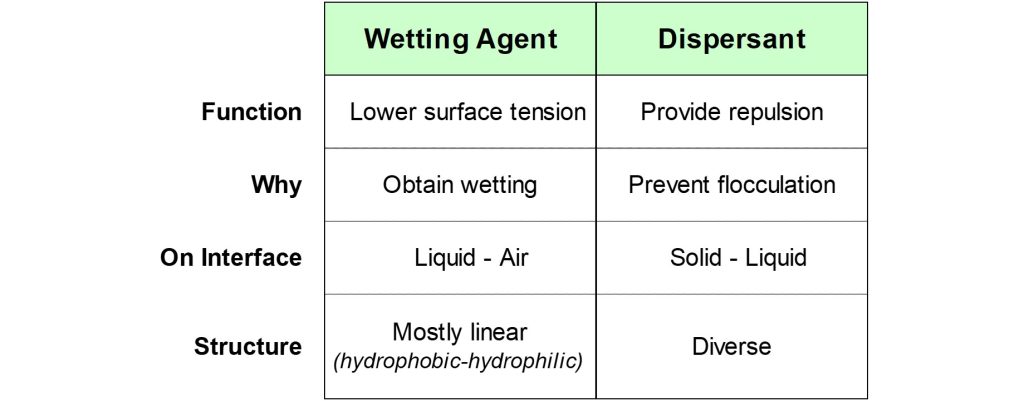
Key differences between wetting agents and dispersants.
System developers, like paint formulators, want to predict how additives will behave in their systems. The behavior of wetting agents and dispersants is governed by their chemical composition and molecular structure. Useful information can be obtained by studying the documentation that describes an additive, like TDS (technical data sheet) and SDS (safety data sheet).
The surface composition of the solid particles that are used, as well as the composition of the continuous liquid phase that surrounds the particles, must be known to be able to select the right additives and to predict the behavior of each additive in a system.
References
- Surface Tension & Surface Energy, Jochum Beetsma, 27 September 2019 (/#)
- Lowering Surface Tension – Surfactants in Coating Materials, Marc Hirsch, 25 February 2021 (/#)
- Dispersant Technology, George Deckner, 25 July 2014. (/#)
- Understanding Dispersants, Marc Hirsch, 19 February 2016 (/#)
The views, opinions and technical analyses presented here are those of the author or advertiser, and are not necessarily those of ULProspector.com or UL Solutions. The appearance of this content in the UL Prospector Knowledge Center does not constitute an endorsement by UL Solutions or its affiliates.
All content is subject to copyright and may not be reproduced without prior authorization from UL Solutions or the content author.
The content has been made available for informational and educational purposes only. While the editors of this site may verify the accuracy of its content from time to time, we assume no responsibility for errors made by the author, editorial staff or any other contributor.
UL Solutions does not make any representations or warranties with respect to the accuracy, applicability, fitness or completeness of the content. UL Solutions does not warrant the performance, effectiveness or applicability of sites listed or linked to in any content.
