 Introduction
Introduction
Dispersion and stabilization of solid particles (pigments and fillers) in a liquid is a challenging process, taking place during the production of paints, colorants and inks. The whole process consists of three steps, starting from dry powdery material that consists of agglomerates of solid particles.
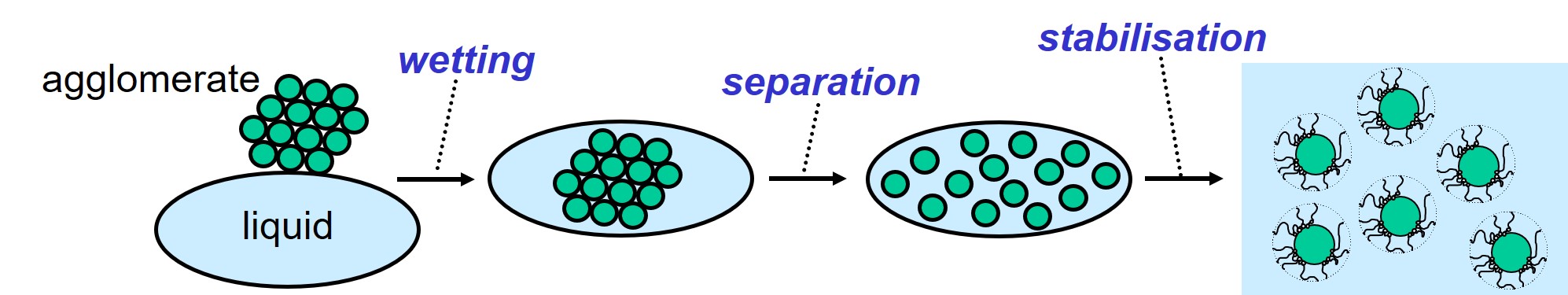
The three steps of the dispersion process.
Wetting of the particles takes place when the powder is brought in contact with liquid. The solid particles are separated from each other by applying mechanical force. The separated particles are stabilized to prevent flocculation. In the following, the three steps are discussed separately even though they mostly occur simultaneously.
- Wetting
All air, that is present within the agglomerates, must be replaced by the liquid that is used in the dispersion process.
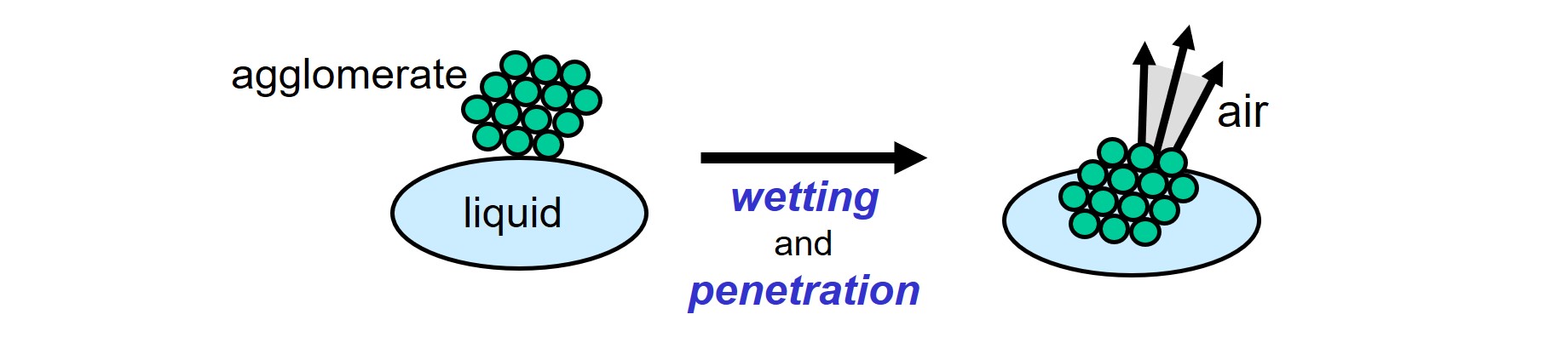
Wetting: solid-air interface is replaced by solid-liquid interface.
A pre-condition for wetting to take place is that the surface tension of the liquid is low enough, compared to the surface energy of the solid particles1. Wetting agents are additives used to obtain wetting in case the surface energy of the particles is too low2,3. The function of a wetting agent is to lower the surface tension of a liquid to such an extent that wetting and penetration of liquid into the capillary channels between the solid particles in an agglomerate, takes place spontaneously. Using wetting agents can induce problems like foam, insufficient intercoat adhesion, increased water sensitivity and reduced film hardness. The good news is that, in many cases, wetting agent is not needed in the dispersion process. Moreover, using wetting agent often retards penetration instead of improving it. Wetting, as well as the release of air from the system, proceeds faster when the viscosity of the liquid is lower. The importance of wetting is often underestimated, thus giving problems in the steps that follow in the dispersion and stabilization process.
Work smarter and win more, with powerful software to manage regulatory, supply chain and sustainability challenges, learn more about ULTRUS here!
- Separation
Agglomerates, consisting of solid particles that are glued together, are split in the separation step. Because of the strong attractive forces between solid particles, separating them requires a high amount of mechanical energy that is provided by suitable equipment.
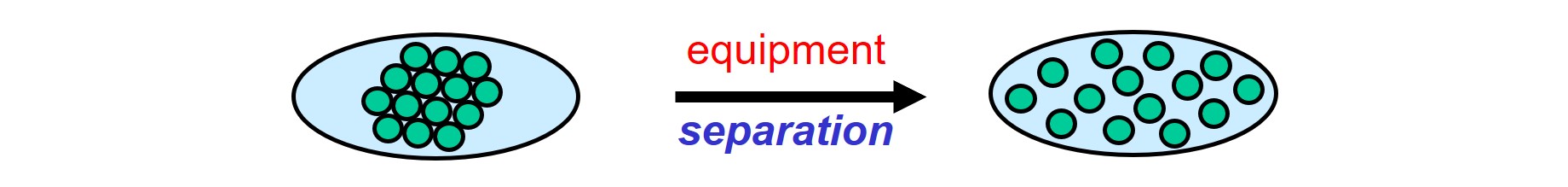
Two principles, shear and impact, are used to split agglomerates into smaller pieces.

A disk disperser, often called dissolver, works by means of shear forces. A disk, with teeth on the edge, rotates with high speed in a liquid mill base of high viscosity, thus applying strong shear forces to the agglomerates.

In a bead mill, often called pearl mill, the impact principle (combined with crunching and shearing) is used to split agglomerates. Beads (pearls) collide with, and move across, each other. Agglomerates, that are between the beads, are hammered, crunched and sheared into smaller pieces.

Mill chamber of a bead mill and beads. (courtesy of WAB)
An axis with rotor blades rotates in the mill chamber that is partly filled with beads. The input of a bead mill is a pre-dispersion that is made by using, for example, a disk disperser. The output of a bead mill is a dispersion of solid particles, that have the desired particle size distribution, in liquid.
- Stabilization
Particles, that have been separated from each other, must be stabilized to prevent flocculation, the spontaneous gluing together of solid particles in a liquid.
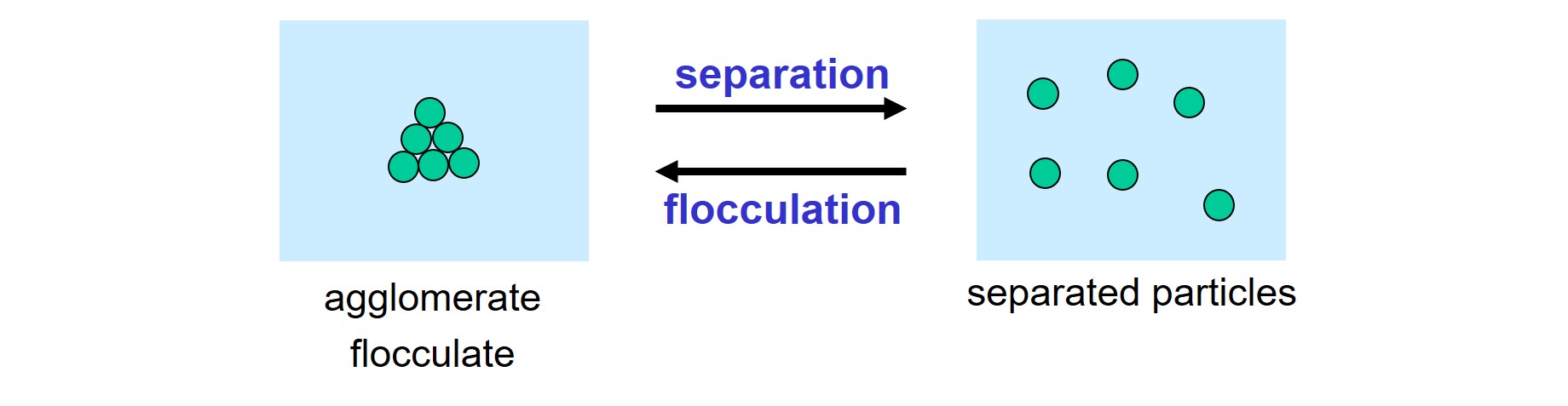
Separation and flocculation of solid particles in a liquid.
Solid particles are stabilized against flocculation by means of a polymeric additive called dispersant4,5,6. Dispersant molecules must adsorb strongly at the surface of the particles. This adsorption phenomenon is often referred to as anchoring. The groups that have a strong affinity for the surface of the particles are called anchoring groups.
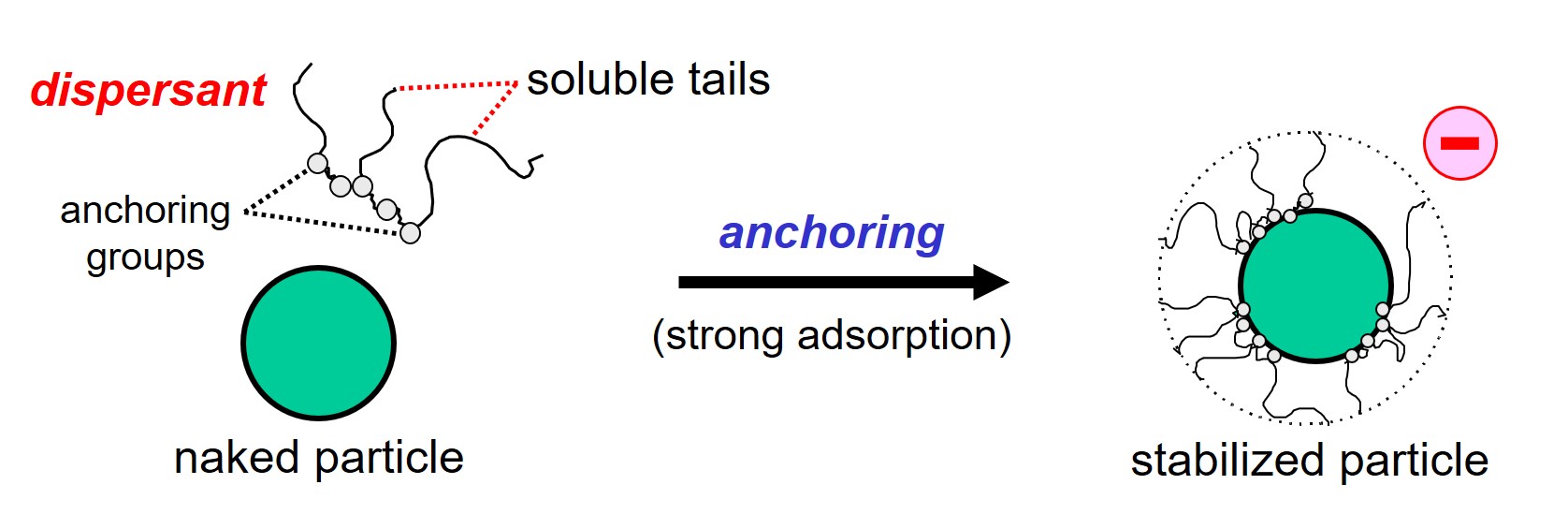
The function of a dispersant, the job the additive must do, is to provide colloidal stability. A dispersion is said to be stable from colloidal point of view when flocculation of separated particles is prevented because the particles repel each other.
Two principles of stabilization against flocculation are used.
First, all particles can have the same electrostatic charge, thus making them repel each other. This so-called electrostatic stabilization7 is critical and it depends upon key system properties like pH and the amount of electrolyte that is present. In waterbased paints and inks mostly anionic stabilization, implying that all particles have a negative charge7, is used.
Secondly, the particles can be covered with a layer of polymeric tails that dissolve in the liquid that surrounds the particles, thus giving steric stabilization8. It turns out that steric stabilization is the most secure principle to use, in both solventbased and waterbased systems.
References
- Surface Tension & Surface Energy, Jochum Beetsma, 27 September 2019.
- Lowering Surface Tension – Surfactants in Coating Materials, Marc Hirsch, 25 February 2021.
- Fundamentals of Wetting, Ron Lewarchik, 26 October 2022.
- Dispersant Agents – How to Avoid Unwanted Consequences, Marc Hirsch, 25 September 2024.
- Dispersant Technology, George Deckner, 25 July 2014.
- Settle Down: Factors that Influence Pigment Settling and Stability, Ron Lewarchik, 12 May 2017.
- Dispersants for Electrostatic Stabilization, Jochum Beetsma, 12 November 2021.
- Dispersants for Steric Stabilization, Jochum Beetsma, 1 October 2021.
The views, opinions and technical analyses presented here are those of the author or advertiser, and are not necessarily those of ULProspector.com or UL Solutions. The appearance of this content in the UL Prospector Knowledge Center does not constitute an endorsement by UL Solutions or its affiliates.
All content is subject to copyright and may not be reproduced without prior authorization from UL Solutions or the content author.
The content has been made available for informational and educational purposes only. While the editors of this site may verify the accuracy of its content from time to time, we assume no responsibility for errors made by the author, editorial staff or any other contributor.
UL Solutions does not make any representations or warranties with respect to the accuracy, applicability, fitness or completeness of the content. UL Solutions does not warrant the performance, effectiveness or applicability of sites listed or linked to in any content.
