Epoxy coatings are widely used in industrial, automotive, marine, and construction applications due to their excellent adhesion, chemical resistance, and durability. Their versatility stems from the chemistry of epoxy resins and curing agents, which allows for tailored formulations to meet specific performance requirements. In this article, we explore the chemistry of epoxy coatings, their key applications, curing mechanisms, and common challenges with troubleshooting strategies.
Activate and energize your sustainability journey, learn more about UL Sustainability Services here!
Chemistry of Epoxy Coatings
Epoxy coatings are primarily based on epoxy resins, which contain reactive epoxide groups, and curing agents (hardeners) that facilitate crosslinking. The most common types of epoxy resins include:
- Bisphenol A-Based Epoxies: The most widely used class, offering excellent mechanical strength, adhesion, and chemical resistance.
- Bisphenol F-Based Epoxies: Provide lower viscosity and improved chemical resistance, often used in high-performance coatings.
- Novolac Epoxies: Offer superior heat and chemical resistance due to higher crosslink density, suitable for extreme environments.
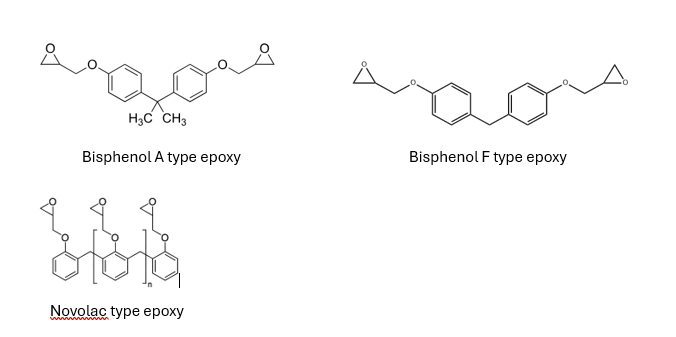
Curing agents play a crucial role in determining the final properties of the coating. Common curing agents include:
- Amine Hardeners: Polyamines, aliphatic amines, and cycloaliphatic amines provide excellent strength and chemical resistance.
- Anhydrides: Slow-reacting but offer enhanced flexibility and thermal stability.
- Polyamides: Impart better flexibility and impact resistance, commonly used in marine and protective coatings.

Key Applications of Epoxy Coatings
Due to their versatility, epoxy coatings are used in various industries, including:
- Industrial and Protective Coatings: Used for corrosion protection in harsh environments such as pipelines, storage tanks, and bridges.
- Floor Coatings: Widely applied in industrial warehouses, hospitals, and garages due to their high wear resistance and easy maintenance.
- Automotive and Aerospace: Employed as primers and topcoats to enhance adhesion and corrosion resistance.
- Marine Coatings: Provide resistance to saltwater, chemicals, and mechanical abrasion for ships and offshore structures.
- Electrical and Electronics: Used as insulating coatings for circuit boards and components due to their excellent dielectric properties.
 Curing Mechanisms of Epoxy Coatings
Curing Mechanisms of Epoxy Coatings
Curing, or crosslinking, is the process where epoxy resins react with hardeners to form a thermoset network. Several factors influence the curing behavior:
- Ambient Temperature vs. Heat-Cured Systems: Room-temperature curing epoxies rely on amine hardeners, while heat-cured epoxies require elevated temperatures for enhanced crosslinking.
- Cure Time and Pot Life: The working time of an epoxy system depends on its formulation, and improper curing can result in underperformance.
- Moisture and Humidity Sensitivity: Some curing agents are highly sensitive to moisture, leading to amine blush (surface contamination that affects adhesion).
Work smarter and win more, with powerful software to manage regulatory, supply chain and sustainability challenges, learn more about ULTRUS here!
Common Problems and Troubleshooting in Epoxy Coatings
Despite their advantages, epoxy coatings can present challenges. Here are some common issues and potential solutions:
- Poor Adhesion
- Cause: Inadequate surface preparation, contamination, or incorrect mixing ratio.
- Solution: Ensure thorough cleaning, proper surface profiling (e.g., sandblasting), and adherence to manufacturer guidelines for mixing and application.
- Blistering and Bubbling
- Cause: Trapped moisture, solvent entrapment, or outgassing from porous substrates.
- Solution: Apply in thin layers, allow solvents to evaporate, and use proper primers to seal porous surfaces.
- Amine Blush
- Cause: Reaction of curing agents with atmospheric CO₂ and moisture, leading to surface haze.
- Solution: Remove with mild detergent and water before applying additional layers.
- Yellowing and UV Degradation
- Cause: Epoxy coatings are susceptible to UV-induced oxidation.
- Solution: Use UV-stable topcoats, such as polyurethane, to prevent discoloration.
- Cracking and Brittleness
- Cause: Excessive film thickness, high crosslink density, or thermal cycling.
- Solution: Adjust formulation with flexibilizers or use appropriate curing conditions to optimize performance.
Latest Innovations and Research Directions
Recent advancements in epoxy coating technology focus on sustainability, enhanced performance, and new applications. Key innovation drivers include:
- Bio-Based Epoxy Resins: To reduce dependency on fossil-derived raw materials, researchers are developing epoxies from renewable sources like lignin, soybean oil, and cashew nutshell liquid.
- Self-Healing Epoxy Coatings: Incorporation of microcapsules or dynamic covalent chemistry enables coatings to repair themselves after damage, extending service life.
- Nanotechnology-Enhanced Epoxies: The addition of nanomaterials such as graphene, silica nanoparticles, and carbon nanotubes improves mechanical properties, thermal stability, and chemical resistance.
- Waterborne and Low-VOC Epoxy Systems: Environmental regulations drive the development of high-performance, solvent-free, and waterborne epoxy formulations to minimize volatile organic compound emissions.
- Advanced Anticorrosion Technologies: Novel epoxy coatings incorporate active inhibitors or smart corrosion-sensing additives to provide better protection for metal substrates.
- Fast-Curing and UV-Curable Epoxies: Research into faster curing mechanisms, including light-activated and hybrid curing systems, is helping to reduce application time and energy consumption.
Drivers for Innovation in Epoxy Coatings
Several factors are pushing innovation in epoxy coating technology:
- Environmental Regulations: Stricter VOC and hazardous substance regulations necessitate more sustainable formulations.
- Performance Demands: Industries such as aerospace, automotive, and marine require coatings with superior durability, chemical resistance, and thermal stability.
- Energy Efficiency: The need for lower curing temperatures and faster application processes drives advancements in curing technology.
- Smart Coatings Development: Emerging applications in electronics, biomedical fields, and protective coatings require multifunctional properties, such as conductivity, antimicrobial activity, and self-repair capabilities.
Conclusion
Epoxy coatings are indispensable in various industries due to their exceptional protective properties and adaptability. Understanding their chemistry, curing mechanisms, and potential application challenges allows formulators and end-users to optimize performance and longevity. Additionally, ongoing research and innovation continue to improve epoxy coatings, making them more sustainable, durable, and multifunctional. By addressing common issues through proper formulation, surface preparation, and environmental considerations, epoxy coatings can achieve maximum durability and effectiveness in demanding environments.
The views, opinions and technical analyses presented here are those of the author or advertiser, and are not necessarily those of ULProspector.com or UL Solutions. The appearance of this content in the UL Prospector Knowledge Center does not constitute an endorsement by UL Solutions or its affiliates.
All content is subject to copyright and may not be reproduced without prior authorization from UL Solutions or the content author.
The content has been made available for informational and educational purposes only. While the editors of this site may verify the accuracy of its content from time to time, we assume no responsibility for errors made by the author, editorial staff or any other contributor.
UL Solutions does not make any representations or warranties with respect to the accuracy, applicability, fitness or completeness of the content. UL Solutions does not warrant the performance, effectiveness or applicability of sites listed or linked to in any content.
