 What are the potential roles of polymers in EV batteries? Andy Pye reviews a report from the UK which looks at battery fire protection materials, and some research from China looking at the potential for polyimides for battery electrodes.
What are the potential roles of polymers in EV batteries? Andy Pye reviews a report from the UK which looks at battery fire protection materials, and some research from China looking at the potential for polyimides for battery electrodes.
Global electric vehicle (EV) sales increased year-on-year by 23% in 2024, despite significant regional disparity. EV fires are less common than those in combustion engine vehicles, but safety cannot be overlooked at any rate of occurrence and test methods and regulations continue to evolve.
Many factors go into the choice of fire protection material, and it will include factors like cell form factor, pack design, thermal management approach, material costs, system costs, regulation to be met, and a wide variety of other factors. Each material has its own trade-offs and needs to be evaluated for each battery design.
Cell form factor
One of the first considerations is the battery cell form factor. In 2024 around 58% of the market was using prismatic cells, followed by 22% cylindrical, and the rest pouch. While the cell form factor may not impact protection at a module or pack level, but it will certainly impact inter-cell materials.
It is common to see sheet-type materials applied between the cells in prismatic or pouch cell systems, with encapsulating foams being a popular choice for cylindrical cell systems. There appears to be no technical reason why this is the case, and both are interchangeable, but it simply reflects common practice.
The failure mode will also vary by cell type, with cylindrical and prismatic cells generally venting through the vents on the top of the cells, which defines the placement of fire protection materials.
Thermal management
A critical factor is how the temperature of the cells is managed. A popular approach is via a cold plate that sits at the top or bottom of the cells through which water-glycol coolant is passed. Thermally insulating cells from one another helps to maintain the temperature within the cells in cold conditions but is also beneficial in preventing heat transfer between a cell in thermal runaway and the rest in the pack.
A second option is to additionally cool the sides of the cells, or use heat-spreading materials between the cells to transfer as much heat away from the cells as possible. Depending on which approach is taken, this will impact what material or combination of materials are chosen to be placed between the cells. For example, foams and aerogels have a very low thermal conductivity, whereas encapsulants or phase change materials tend to have a higher thermal conductivity.
EV Battery Testing for Compliance with Regulatory Requirements and Standards, learn more here!
Material choices
Even once the cell form factor and thermal management strategy are decided, many different material classes and chemistries can be considered.
IDTechEx has published a report entitled “Fire Protection Materials for EV Batteries 2025-2035: Markets, Trends, and Forecasts”. Materials discussed include ceramic blankets/sheets, mica, aerogels, coatings, encapsulants, encapsulating foams, compression pads (with fire protection), phase change materials, and polymers (with fire protection). Encapsulating foams and mica currently dominate material demand.
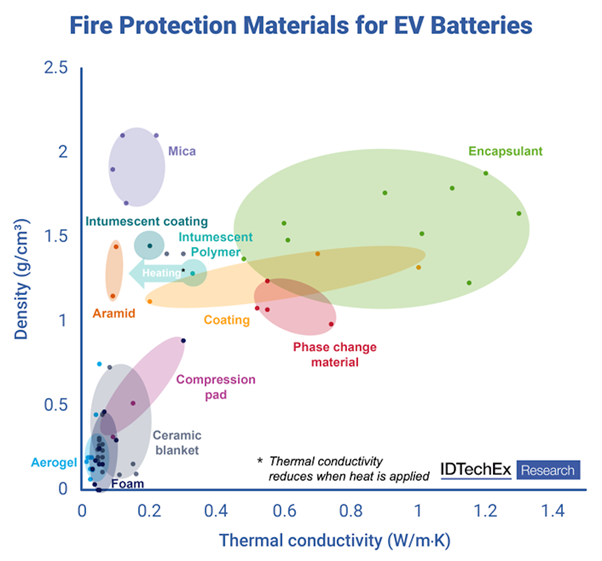
Mica is a very low-cost material with strong electrical insulation properties, but is usually required in thicker (and heavier) layers to provide sufficient fire protection.
On key metrics like low thermal conductivity and density, aerogels, ceramic blankets, and encapsulating foams do perform well. However, this does not tell the entire story: often, a combination of materials may be needed to meet structural requirements and/or meet UL2596 test requirements. UL2596 tests are challenging, with the torch and grit test exposing the sample to an alternating high-temperature flame (1200°C) and alumina particle blasts; meeting them will typically increase the density required.
Of course, a primary concern for battery designers is system cost. The addition of fire protection materials increases costs, which can vary widely between material options. Part of the calculation is determined by how much of each material is required to achieve the required performance. James Anderson, report author and research director at IDTechEx, predicts that newer emerging options will start to eat into this market share. Some of these newer options include aerogels, and intumescent polymers and coatings.
Aerogels have historically been limited by cost, but in the right implementation they can provide excellent thermal insulation and fire protection. As mentioned above, if a material can provide multiple functionalities, then its additional costs can reduce its impact on overall system cost.
Accordingly, suppliers are trying to produce materials that perform multiple functions and can prevent the need for multiple assembly steps. An increasingly popular option from suppliers is polymer/composite components with fire protective and/or intumescent properties that can be made into structural components of modules, cell holders, or an inter-cell spacer. These may be less thermally insulating and of higher density than some other candidates in normal operation, but they can replace the need for multiple other materials, while providing good insulation at high temperatures. Examples of companies active in this field include SABIC, Pyrophobic Systems and Asahi Kasei.
Polyimide materials for next-generation batteries
Organic electrode materials are promising candidates for next-generation energy storage systems. However, challenges such as limited active-site density and inefficient utilization have impeded their practical application. Addressing these hurdles requires a deeper understanding of how molecular structure impacts electrochemical performance — an area where significant progress is now being made.
Innovative research at Jilin University and the Chinese Academy of Sciences demonstrates how incorporating carbonyl and conjugated structures can transform lithium-ion battery performance, providing a roadmap for future advancements.
By addressing key limitations of organic cathodes, the work has profound implications for the future of lithium-ion battery technology. Advanced polyimide cathodes are well-suited for applications in portable electronics, electric vehicles, and large-scale energy grids.
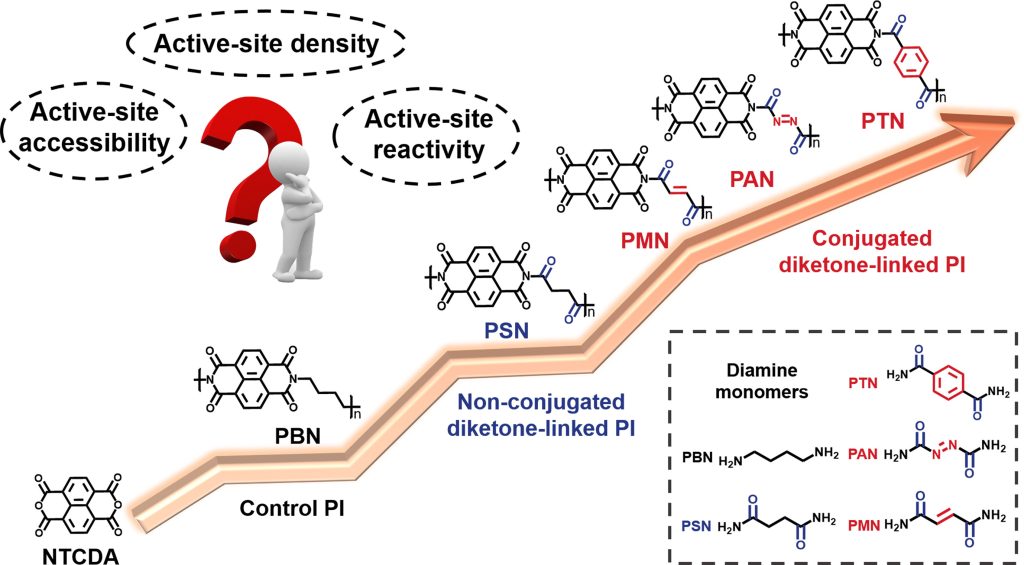
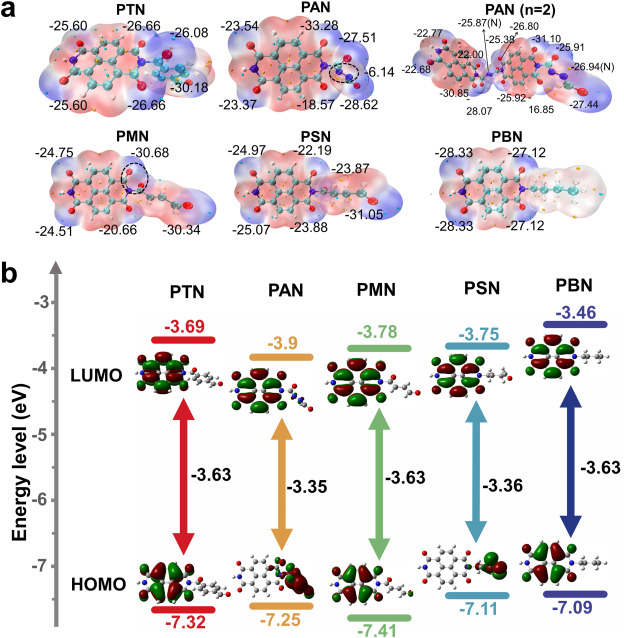
The researchers synthesized and tested five polyimides — PTN, PAN, PMN, PSN and PBN — each engineered with distinct molecular modifications to improve performance. Although they share a similar core (naphthalimide units), each variant used a different diamine linker to introduce more carbonyl “hot spots” and adjust the polymer’s rigidity. It turns out both factors — how many Li-ion–friendly carbonyl sites each molecule has, and how neatly its backbone is arranged — are pivotal in pushing battery capacity over the 200 mAh/g threshold.
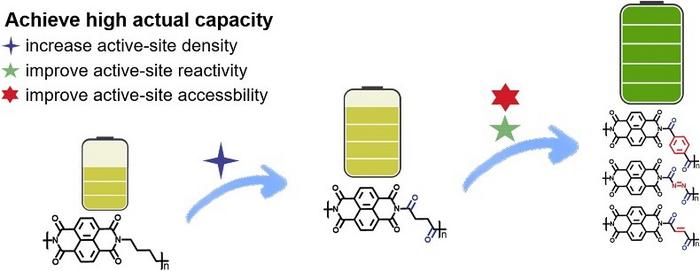
PTN came out on top, providing a 212 mAh/g capacity at 50 mA/g, thanks to a well-tuned ratio of accessible carbonyl groups and a rigid backbone that curbed self-stacking, helping electrons move swiftly. Across the board, modifying these molecular structures also boosted other key metrics such as the rate performance — how well the batteries operate under higher current loads — and cycle life.
All polyimides displayed impressive cycling stability, with capacity retention rates exceeding 97% across extended use, showcasing the robustness of this molecular engineering approach. These findings illuminate new pathways for designing high-performance organic electrode materials.
Discover Material Selection with Prospector Premium, learn more here!
Polyimides
Polyimide (PI) is a high-performance polymer with a ring bond in its molecular chain. It is known for its thermal stability, chemical resistance, and mechanical properties. Polyimides were originally developed by the DuPont Company. Kapton is a classic polyimide. Polyimides have excellent dielectric properties and an inherently low coefficient of thermal expansion. They can withstand extreme temperatures without significant degradation.
Applications
- Coatings and film substrates
- Electrical devices: fuel cells, displays, and other electrical devices.
- Automotive
- Insulation coatings
- High temperature structural adhesives: Polyimide is used in high-temperature structural adhesives.
- Waveguides
Polyimide variants used in this research programme
PBN – this common polyimide contains a redox-inert alkyl synthesized from NTCDA and 1,4-butanediamine without redox sites. It was used as a control structure, as it lacks those extra carbonyl sites and sports a more flexible chain.
PSN – compared to PBN, the specific capacity of polysimizane (PSN) significantly increases with the introduction of carbonyl groups, although the two C=O groups of the diamine moieties for PSN are separated by a non-conjugated ethyl structure. Polysilazane is often used as a coating with polyimides to create high-carbon ceramic and gas barrier films.
PTN – IVL’s polytrimethylene naphthalate (PTN) is a specialty polyester polymer derived from 1,3-propanediol plus either NDC or PNDA. It is structurally analogous to polytrimethylene terephthalate (PTT), but with improved thermal, barrier (both diffusional and UV penetration), mechanical, and weathering properties.
PAN – polyacrylonitrile (PAN), is a synthetic polymer often used in fibre production. It is a rigid, crystalline polymer that has a high melting temperature and a high modulus of elasticity. It has excellent impact resistance and is resistant to a wide range of chemicals, including acids, bases, and organic solvents.
PMN – a specific type of polyimide polymer where “PMN” stands for “pyromellitic dianhydride,” which is a key chemical component used in its synthesis. PMN is often used in applications requiring high thermal stability and mechanical strength due to its rigid backbone structure.
References
- J Anderson. Fire Protection Materials for EV Batteries 2025-2035: Markets, Trends, and Forecasts
https://www.idtechex.com/en/research-report/fire-protection-materials-for-ev-batteries-2025-2035-markets-trends-and-forecasts/1068 - Jun Wang et al. Towards high performance polyimide cathode materials for lithium–organic batteries by regulating active-site density, accessibility, and reactivity
https://doi.org/10.1016/j.esci.2023.100224
The views, opinions and technical analyses presented here are those of the author or advertiser, and are not necessarily those of ULProspector.com or UL Solutions. The appearance of this content in the UL Prospector Knowledge Center does not constitute an endorsement by UL Solutions or its affiliates.
All content is subject to copyright and may not be reproduced without prior authorization from UL Solutions or the content author.
The content has been made available for informational and educational purposes only. While the editors of this site may verify the accuracy of its content from time to time, we assume no responsibility for errors made by the author, editorial staff or any other contributor.
UL Solutions does not make any representations or warranties with respect to the accuracy, applicability, fitness or completeness of the content. UL Solutions does not warrant the performance, effectiveness or applicability of sites listed or linked to in any content.
