 Interfaces, boundaries between two phases, are important in paint and ink technology. The behavior of a system during production, storage, application and film formation is, to a large extend, governed by the composition of the interfaces that are present and by the amount of interfacial area. All interfaces are thermodynamically unfavorable, but some interfaces are more unfavorable than others. Interfacial energy (given in J/m2), also called interfacial tension (given in N/m), is the first property that governs how unfavorable an interface is. It is associated with the composition of the interface. The second property is the surface area of the interface, given in m2. Two categories of interfacial energy, represented by the Greek symbol γ (‘gamma’), are most important in paints and inks.
Interfaces, boundaries between two phases, are important in paint and ink technology. The behavior of a system during production, storage, application and film formation is, to a large extend, governed by the composition of the interfaces that are present and by the amount of interfacial area. All interfaces are thermodynamically unfavorable, but some interfaces are more unfavorable than others. Interfacial energy (given in J/m2), also called interfacial tension (given in N/m), is the first property that governs how unfavorable an interface is. It is associated with the composition of the interface. The second property is the surface area of the interface, given in m2. Two categories of interfacial energy, represented by the Greek symbol γ (‘gamma’), are most important in paints and inks.
Surface tension
Surface tension is a property of liquids that is governed by intermolecular interactions: it originates from the cohesive, the attractive, forces between molecules in a liquid. Thermodynamics tells us that systems, like paints and inks, strive to attain a state with a maximum number of favorable interactions. This implies that liquids will shape in such a way that the number of bulk molecules is at a maximum and the amount of surface molecules is at a minimum.
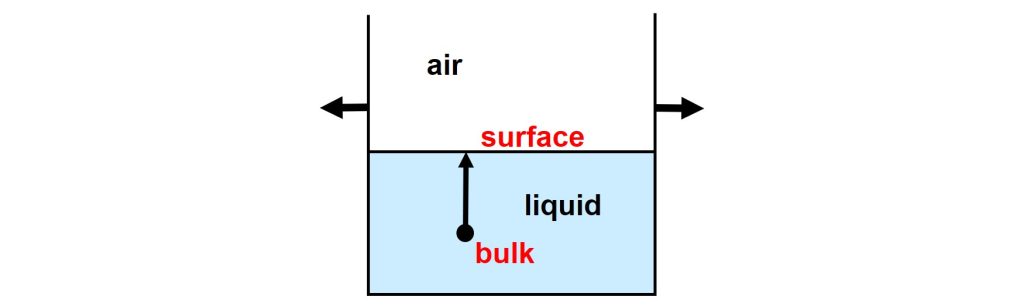
Molecules at the surface of a liquid are not fully surrounded by their fellows; surface molecules are partly naked. This implies that molecules at the liquid-air interface experience less favorable interactions and are therefore in a state of higher energy, compared to molecules in the bulk of the liquid.
Therefore, energy is needed to move molecules from the bulk of the liquid to the surface. The stronger the interactions between the molecules are, the more energy is required to increase the surface area of a liquid.
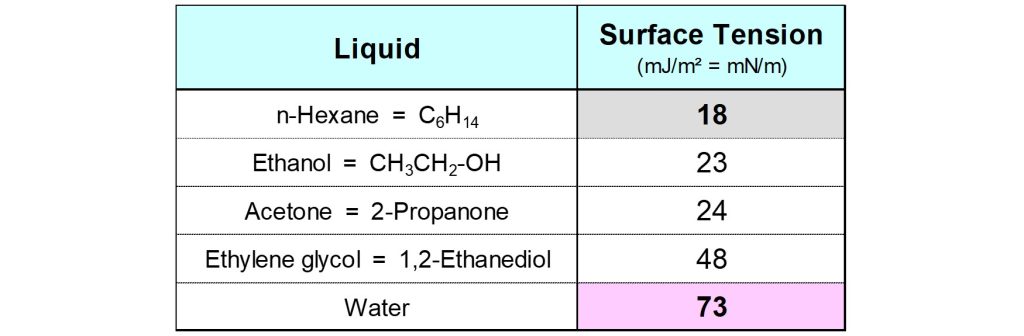
The surface tension of a liquid, γlg , is defined as the energy (in Joule) needed to create 1 m2 of new liquid-gas interfacial area. The dimension of γ is J/m2. Often the dimension N/m (Newton per meter) is used. Two subscripts are used to specify the interface to which an interfacial energy refers. An interface is a boundary between two phases. Therefore, the abbreviation of these phases should be included as subscript in the symbol for the interfacial energy. Surface tension is the interfacial energy of a liquid-gas interface. In paints and inks the ‘gas’ phase is most often the air above the system.
Work smarter and win more, with powerful software to manage regulatory, supply chain and sustainability challenges, learn more about ULTRUS here!
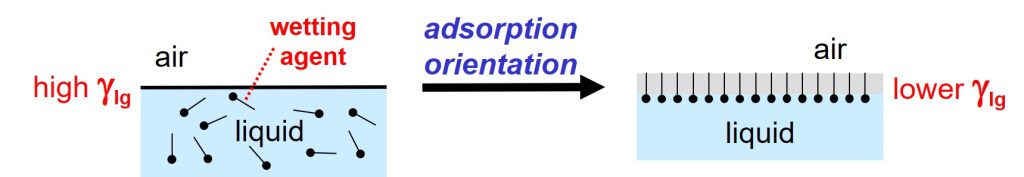
n-Hexane has a low surface tension because the mutual interactions between the molecules in the liquid are relatively weak. The surface tension of a pure liquid with high surface tension, like water, can be lowered by adding molecules that have a surfactant structure, like wetting agents.1 Such additives lower the surface tension of a liquid because the molecules adsorb and orient at the liquid-air interface in such a way that the hydrophobic tails point towards the air and the hydrophilic parts stick into the liquid. Wetting can be improved, and film defects can be prevented, by using suitable wetting agents.2
Surface energy
Surface energy is a key property of solid materials, like substrates on which paint must be applied. Surface energy is the interfacial energy of a solid-gas interface. The property is represented by the symbol γsg, with the subscript ‘s’ for solid and ‘g’ for gas. The gas above the solid surface is most often air.
Strong interactions are possible with a solid surface that has a high surface energy. This implies that a high surface energy is beneficial for the wetting of, and adhesion on, substrates. Solids with a low surface energy, like most untreated plastics, are difficult to coat.
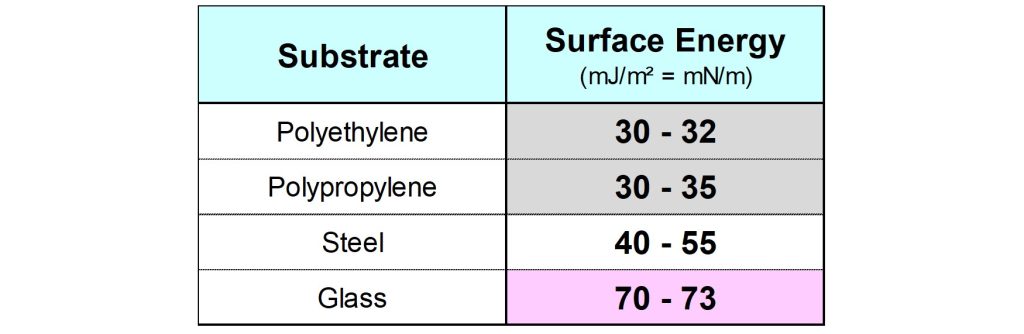
The surface energy of plastic objects can be raised by using pre-treatment techniques, such as corona treatment, plasma treatment or flame treatment, thus improving both wetting and adhesion.3
References
- Classifying Surfactants for use in Coatings Formulation, Marc Hirsch, 28 May 2021
- Fundamentals of Wetting, Ron Lewarchik, 26 October 2022.
- Plasma Processing of Plastic Surfaces, 30 December 2016, Andy Pye.
The views, opinions and technical analyses presented here are those of the author or advertiser, and are not necessarily those of ULProspector.com or UL Solutions. The appearance of this content in the UL Prospector Knowledge Center does not constitute an endorsement by UL Solutions or its affiliates.
All content is subject to copyright and may not be reproduced without prior authorization from UL Solutions or the content author.
The content has been made available for informational and educational purposes only. While the editors of this site may verify the accuracy of its content from time to time, we assume no responsibility for errors made by the author, editorial staff or any other contributor.
UL Solutions does not make any representations or warranties with respect to the accuracy, applicability, fitness or completeness of the content. UL Solutions does not warrant the performance, effectiveness or applicability of sites listed or linked to in any content.
