Introduction
Excellent adhesion on the substrate is a key requirement for most coating systems. The quality of the substrate-coating interface, and by that the adhesive strength, is governed by the quality of the paint, the condition of the substrate, the application process and film formation.
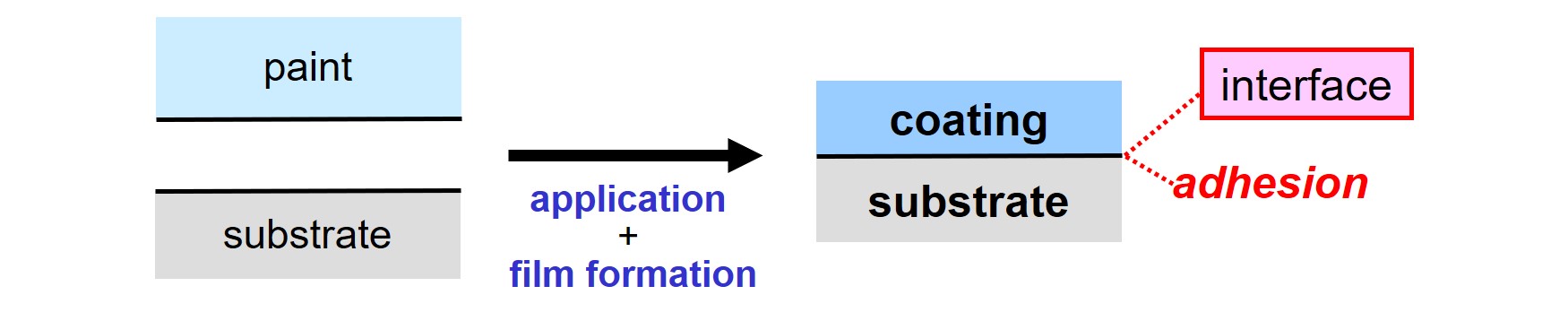
Several mechanisms can be used to obtain adhesion1,2. This article is about adhering coatings on steel via covalent bonding.
The important start
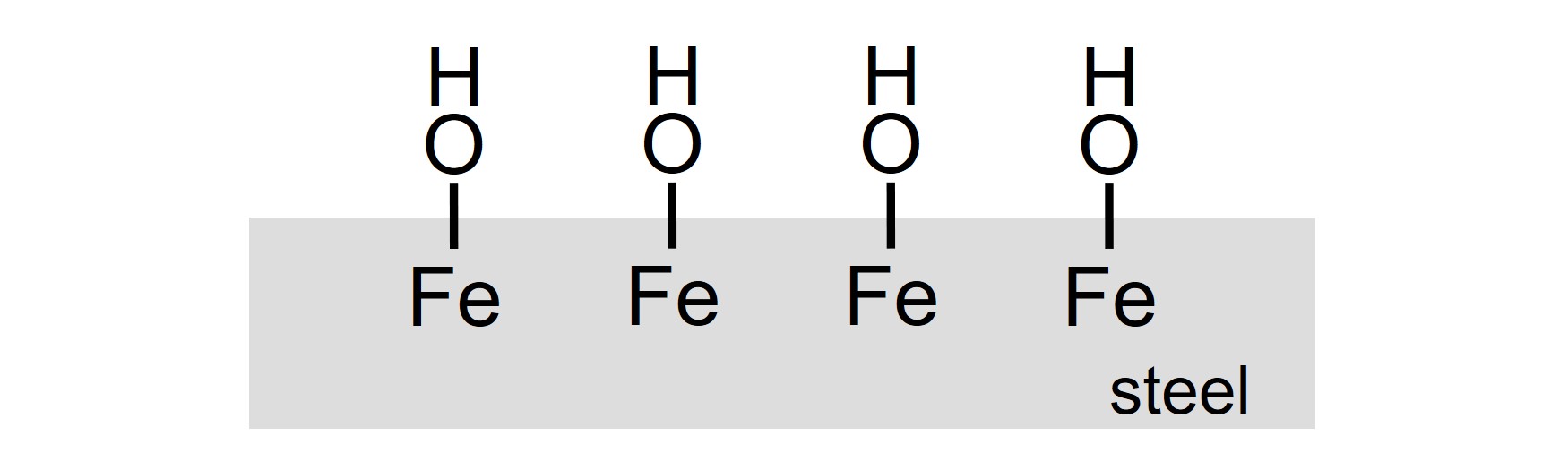
The surface of a steel object that is coated must be pure and clean. This implies that oxidation products of iron, like rust and scale, must be removed before paint is applied. Also, the substrate must be cleaned, to remove dirt, grease et cetera, for example by using alkaline cleaner. After cleaning, the surface must be rinsed and dried. A steel surface, that is filled with hydroxyl (–OH) groups, results when the pre-treatment is done right.
Key success factors
An approach to obtain excellent adhesion of a coating on steel is to assure interfacial crosslinking. This implies that covalent chemical bonds, that are resistant against hydrolysis, are formed between the steel surface and the coating during film formation. The surface of pure and clean steel is filled with reactive hydroxyl groups, thus enabling interfacial crosslinking. Apart from requirements with respect to the substrate and the paint themselves, the resulting steel-coating interface must be flexible enough to cope with dimensional changes that are caused by changing temperature or mechanical stress, possibly in combination with the exposure to water.
Work smarter and win more, with powerful software to manage regulatory, supply chain and sustainability challenges, learn more about ULTRUS here!
Adhesion promoters
Adhesion promoters, also known as coupling agents, are bi-functional reactive additives that are designed as such that one part of the molecules forms covalent bonds with the substrate and another part of the molecules participates in the crosslinking of the binder system of the paint during film formation3,4. Bi-functional silanes, based on silicon (Si) chemistry, are often used as adhesion promoters for steel.
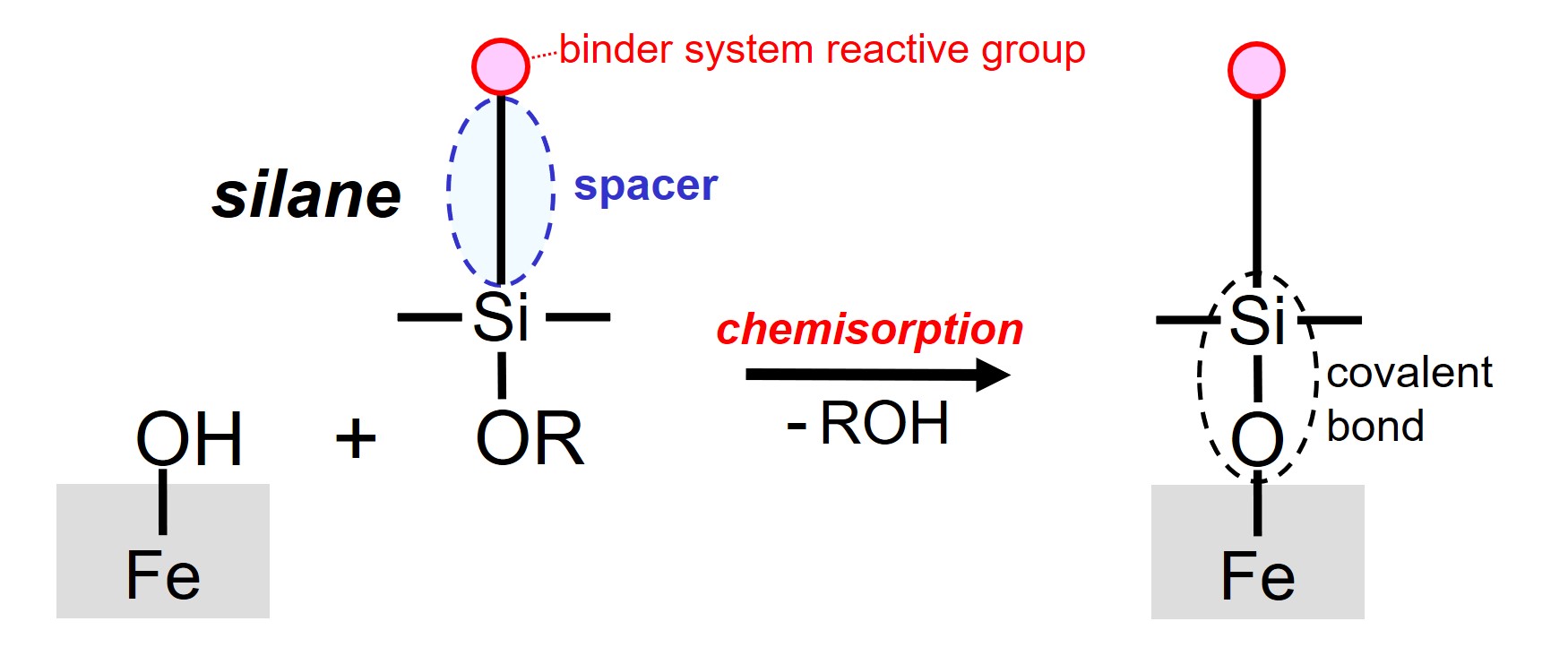
A chemical reaction of one part of the silane adhesion promoter with a hydroxyl group on the steel surface takes place when the adhesion promoter molecule comes in contact with the steel under the right conditions. During this so-called chemisorption, an alcohol molecule (R-OH) is released and a covalent bond (Fe-O-Si) is formed.
The chain between the two types of reactive groups, the spacer, is designed as such that the resulting substrate-coating interface is flexible enough, thus preventing possible stress building-up at the interface. It is said that the spacer gives interfacial flexibility.
An example
3-Aminopropyltriethoxysilane (APTES) is a molecule that can be used as adhesion promoter to couple a 2-component (2C) primer with a steel surface.
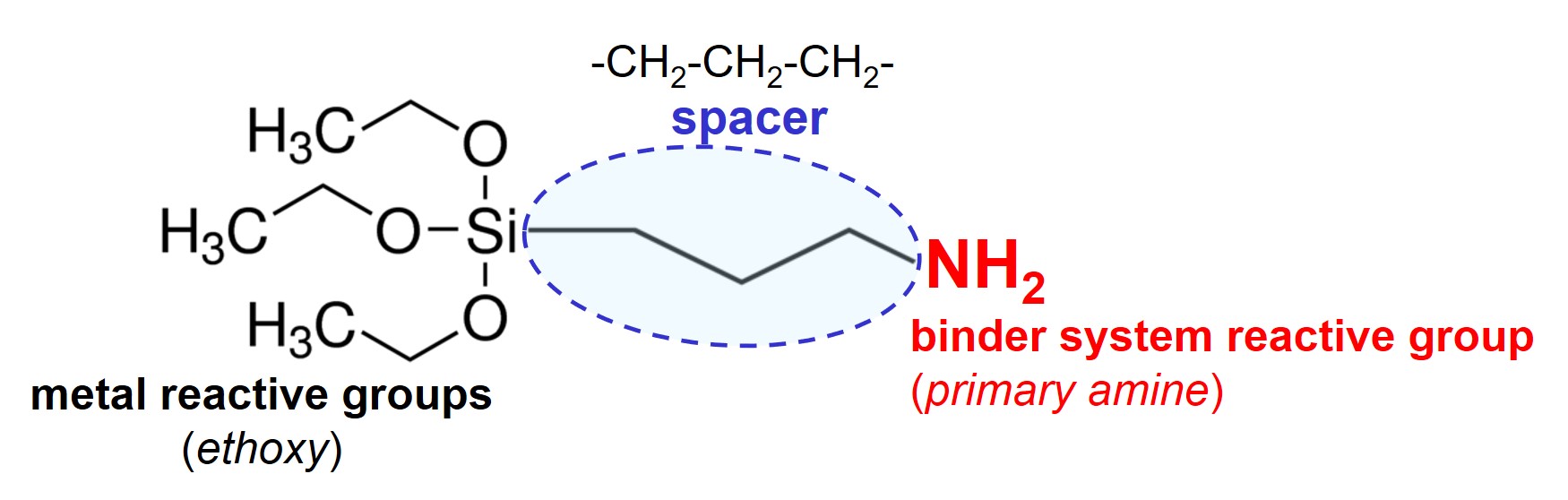
The metal reactive block, consisting of three ethoxysilane groups, can react with hydroxyl groups on the steel surface under the release of ethanol (H3C-CH2-OH). The primary amine group (–NH2) is reactive towards both epoxy groups and isocyanate groups. This implies that APTES can be used as adhesion promoter for both 2C epoxy-amine systems and 2C polyurethane systems.
Let’s take a closer look at the APTES molecule. The three metal reactive groups are close to each other. This is critical with respect to both reactivity and flexibility. The second critical aspect is that the molecules contain only one binder system reactive group. Finally, the spacer has a critical length of only 3 methylene (-CH2-) units, providing limited interfacial flexibility.
Oligomers
System developers recognize the critical aspects of conventional adhesion promoters like APTES. Because of that, monomeric silanes are more and more substituted by oligomeric adhesion promoters. An oligomer is defined as a polymer with a relatively low molecular weight5.
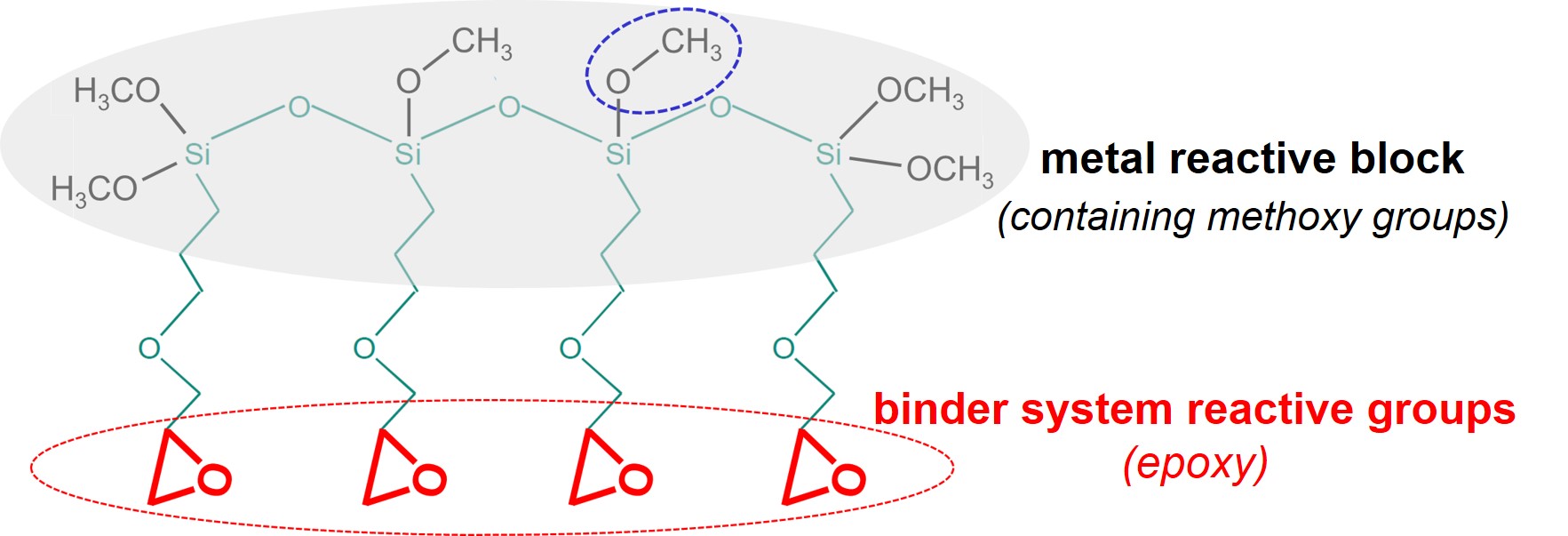
CoatOSil MP 200 is an example of an oligomer, based on silane chemistry, that can be used as adhesion promoter for 2C metal primers that are based on epoxy-amine binder systems.
The metal reactive block contains several methoxysilane groups (Si-O-CH3) that can react with hydroxyl groups on the steel surface, under the release of methanol (HO-CH3) molecules. Each molecule has multiple epoxy groups, thus enabling multiple co-crosslinking with the binder system of the primer. Finally, the spacers in MP 200 are more flexible than the spacer in APTES, giving both increased reactivity and more interfacial flexibility.
Application
Basically, there are two options with respect to the use of adhesion promoters.
First, a dilution of adhesion promoter can be applied directly on the clean steel surface. In this way, a thin reactive layer is formed on the steel surface, a so-called conversion layer: the steel surface is converted from hydroxyl-functional to epoxy-functional or amine-functional. The next step is the application of the paint on the modified surface in such a way that the binder system of the paint forms covalent bonds with the conversion layer.
The second option is to use the adhesion promoter as additive in the paint that is applied on the steel. This can, for example, be a primer based on a 2C epoxy-amine binder system or a primer based on a 2C polyurethane binder system. A critical factor of the second approach is that an undesired pre-reaction of the adhesion promoter molecules with paint ingredients can take place before the paint is applied on the steel. Also, the adhesion promoter molecules must be able to migrate from the bulk of the paint to the steel-paint interface during film formation. Both critical factors imply that the best option is to first apply the adhesion promoter as conversion layer before paint is applied. The disadvantage of this approach is that an extra application step is needed.
References
- A Guide to Providing Initial and Long-Lasting Coating Adhesion, Ron Lewarchik, 12 January 2022
- Achieving Superior Adhesion of Coatings, Jochum Beetsma, 13 November 2020
- Superior Coatings Performance with Organosilane Components, Ron Lewarchik, 10 August 2018
- Adhesion Promoters 101, Marc Hirsch, 9 November 2022
- Oligomers – Small Group, Big Impact, Wally Kesler, 27 September 2023
The views, opinions and technical analyses presented here are those of the author or advertiser, and are not necessarily those of ULProspector.com or UL Solutions. The appearance of this content in the UL Prospector Knowledge Center does not constitute an endorsement by UL Solutions or its affiliates.
All content is subject to copyright and may not be reproduced without prior authorization from UL Solutions or the content author.
The content has been made available for informational and educational purposes only. While the editors of this site may verify the accuracy of its content from time to time, we assume no responsibility for errors made by the author, editorial staff or any other contributor.
UL Solutions does not make any representations or warranties with respect to the accuracy, applicability, fitness or completeness of the content. UL Solutions does not warrant the performance, effectiveness or applicability of sites listed or linked to in any content.
